Regulations
The risk classes of medical devices
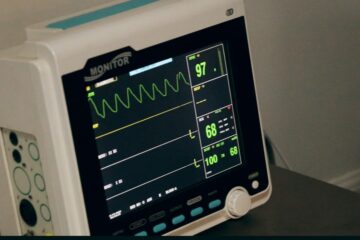
How MDs are classified in America and Europe according to the risks they are subject to. In both America and Europe, medical devices are classified based on the level of risk they pose to patients and users. The classification criteria vary slightly between the two regions, but generally, the higher Read more…